RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM STATUS IN CHILDREN WITH MYOCARDIAL PATHOLOGY WITH VARIOUS LEVELS OF RIGHT VENTRICULAR FUNCTION
Дата: 2013/5/15 | Раздел: Современная педиатрия
L.F. Bogmat, Рў.Рђ. Golovko
The Nat. Acad. Med. Sci. Institute for Children and Adolescent Health Protection State Institution, Kharkiv, Ukraine
UDC 616.123:616.12_008.46_053.6:577.17
The objective of the study: to research the status of rennin-angiotensin-aldosterone system in children with myocardial pathology at various levels of right ventricular function. 109 patients with myocardial pathology (recurrent cardiomyopathy, dysplastic cardiomyopathy, arrhythmias) were studied. Clinical studies with analyses of complaints, disease history, life history and objective research data were carried out. The study into the RAAS system included studies of plasma renin activity, angiotensin II and aldosterone levels in peripheral venous blood through radioimmunological analysis carried out with NarkoTest gamma meter. The morphofunctional status of the right ventricle was assessed based on results of ultrasound Doppler examination of the heart in M and B modes. It was established that in children with MP systolic dysfunction of the RV and it deconditional remodelling form and progress on the background of neurohumoral regulation systems and in particular, the RAAS activation.
Keywords: rennin-angiotensin-aldosterone system, right ventricle of heart, children, myocardial pathology.
Introduction
In recent years it has been proven that a disorder of metabolic processes in the myocardium with its subsequent dysfunction is the most frequent releaser for development of early manifestations of chronic heart insufficiency (CHI) [7]. It causes the start of compensatory response aimed at maintaining adequate perfusion pressure in vessels by increasing heart's propulsive capacity, increasing the vasomotor tone (peripheral vasoconstriction) and increasing the circulatory blood volume (CBV) [3]. Herewith, a sequential activation of neurohumoral systems — first, of the sympathoadrenal system (SAS) and natriuretic peptides (NUP); then, on a later, though still pre-clinical, stage, of the rennin-angiotensin-aldosterone системы (RAAS) — happens [4].
Until recently the RAAS has been considered just as a circulatory neuroendocrine system; however, it has been showed of late [8, 9] that, on top of circulatory components, its so-called local, or tissue, components are of great functional importance. The circulatory RAAS is a complex ferment and hormonal system the main components of which are renin, angiotensinogen, angiotensin I, angiotensin converting enzyme (ACE), angiotensin II, aldosterone and their specific receptors.
The process of renin release is stimulated by a number of factors though mostly, by increased sympatic activity, reduced renal blood circulation along with reduction of renal perfusion pressure and negative sodium balance [4]. Already at minimal systolic dysfunction in the left ventricle the hormones and mediators of the vegetative nervous system acting immediately via n.vagus and by impacting on the renal pres-so-sensitive mechanism increase renin secretion in cells of the juxtagromerular mechanism and its release into the bloodstream. Renin contributes to angioten-sinogen transformation into angiotensin I and in parallel activates angiotensin transforming enzyme that, in its turn, assists in angiotensin I transformation into angiotensin II.
The latter is the main effector peptide of the RAAS that, in its turn, intensifies noradrenalin secretion from sympatic neurons and thus contributes even more to the increase of sympatic activity; it also stimulates aldosterone secretion in the zona glomerulosa of the adrenal cortex [1]. Aldosterone is a mineralcorticoid that at its increased secretion causes haemodynamic shifts through the increase of intravascular volume, sodium retention and negative potassium balance.
RAAS activation at CHI is aimed at optimization of interrelation between cardiac output with peripheral vascular resistance and CBV. However, prolonged extra activity of the system on the background of haemodynamic disorders leads to pronounced sodium and water retention and higher levels of extracellular fluid that, in its turn, contributes to pathological redistribution of electrolytes and water and disruption of the water and electrolyte homeostasis [3].
Angiotensin-II Рё aldosterone are also powerful 0 fibroblast growth-stimulating factors and as such, stimulate myocardium hypertrophy development. In the same time, the increase in their activity is associated with intensification of cardyomyocyte apoptosis processes because of which activation of myocardiac RAAS is deemed one of the factors that conditions myocardium deterioration, elaboration of cardiac cavity dilatation and progressive degradation of heart pumping function [3].
The processes of left heart remodelling on the background of alterations in neurohumoral regulation systems in patients with various cardiovascular diseases are the best described to date [2,5]. Meanwhile, the venous heart processes are still poorly known.
The objective of the current study was to investigate the status of the rennin-angiotensin-aldosterone system in children with myocardial pathology at various levels of the right ventricular function.
Study Material and Methods
109 patients (66 boys and 26 girls from 11 to 18 y. o.) with various heart diseases were examined; of them, 50 had secondary cardiomyopathy, 28 had heart rhythm and conductivity disorders and 31, dysplastic cardiomyopathy. The control group was made up of 39 their apparently healthy peers, of them 25 boys and 14 girls.
Clinical studies with analysis of complaints, disease and life history and objective examination data were held. The study of the RAAS system included researches into plasma renin activity (PRA), angiotensine II (Рђ-II) and aldosterone (ALDO) levels in peripheral venous blood using the radioimmunological analysis with NarkoTest gamma meter. For the analysis purposes Angiotensin-I-Renin, Angiotensin-II and Aldosterone kits by IMMUNOTECH Company (Czech Republic) were used.
Blood sampling was carried out in the fasted state in horizontal position from the cubital vein of the adolescent after 20 min-long relaxation period and into pre-cooled test glasses. The test glasses had EDTA-Na2 (0.15 ml 6% solution per 10 ml blood) anticoagulation agent added in advance. The test glasses with blood were put in the cold place (on ice), then centrifugated at low t (0°С to -4°С) at 3,000 rpm for 15 minutes. The plasma was stored in the refrigerator at t -28°С. Plasma thawing before analysing was carried out with due regard to its temperature that should not exceed +4°С. Only one-time thawing was allowed.
The functional status of the myocardium was assessed based on echocardiography test (UCG) in M and B modes as well as Doppler echocardiography in continuous wave and colour scan by 5 MHz convex sensor using the Ultrasound Digital Scanner SA-8000 LС–ve (Medison, Korea) following the standard technique recommended in the American Society of Echocardiography's Guidelines. The heart structures were registered per five standard deflections.
To assess morphofunctional parameters of the right ventricle (RV), the following indicators were used: dimensions of the right ventricular outflow track (RVOT), RV end-diastolic and end-systolic dimensions (RVEDD, RVESD), RV end-diastolic and end-systolic volume (RVEDV, RVESV), RV myocardium wall (RVMW), RV ejection fraction (RVEF), RV stroke volume (RVSV), RV minute blood volume (RVMBV). In order to level out age-related fluctuations of RV's linear and volume characteristics, index indicators to relate them with the body surface were calculated (IRVEDD, IRVESD, IRVEDV, IRVESV, IRVSV).
Statistical processing of the obtained data was carried out using Statgrafіcs-5 software package on ІBM PC/Pentіum-4. At the first stage, the arithmetic mean and standard mean square error values for all indicator averages were calculated. For normal distribution of indicators, deviations between the averages were assessed with parametric (Student t test, Fisher angular transformation) math statistical methods and in absence of such normal distribution, with non-parametric (Wilcoxon—Mann—Whitney) math statistical methods. Correlation analysis was used to determine cause-and-effect relationship between morphofunctional RV indicators and RAAS activity indicators. Pair correlation coefficient and rank correlation were calculated.
Study Results and Discussion
The main group included children with light manifestations of myocardium pathology (MP) who in 63% cases complained of short-lived lancinating cardialgias that would happen at any time of the day unrelated to any physical activity. Some of them also complained of heart palpitation episodes (13%), sense of discomfort in the heart area (15%) and rapid fatigability (28%).
Depending on their RV function, children in the main group were split into three subgroups. The first comprised 20 (18.34%) patients with RVEF below 50.0%; the second had 36 (33.03%) patients with RVEF in the range between 51.0% and 60.0%; and the third group comprised 53 (48.62%) patients with RVEF above 61.0%. Each of the subgroups showed certain specific differences in morphofunctional parameters of the right ventricle (Table 1).

Specifically, a reduction of RV diastolic dimension registered in children with RVEF above 61.0% on the background of increased functional capacity of the myocardium (EF, ÄS, SV) with parallel reduction of the systolic dimension and volume (Table 1) may serve an indirect indication of possible diastolic dysfunction formation.
These processes go deeper in the second subgroup of children with RVEF from 50.0% to 60.0%. We see further reduction of RV's end-diastolic dimension offset by an increase in its end-systolic dimension and volume and verifiable deterioration of systolic function parameters (ISV, RVEF, and ÄS). The sufficiently high RVMBV level is retained owing to higher HR (Table 1).
In children with less than 50.0% RVEF, a considerable deterioration of the myocardium pump function (SV, RVEF, ÄS) with verifiable increase of end-systolic dimension and volume index indicators has been registered. RVMBV also decreases because of a pronounced stroke volume reduction (Table 1). The diastolic dimension and volume were no different from the previous group.
The presented alterations in RV morphofunctional parameters in children with MP attest to these children suffering from deconditioned remodelling of this part of the heart with subsequent formation of its systolic dysfunction.
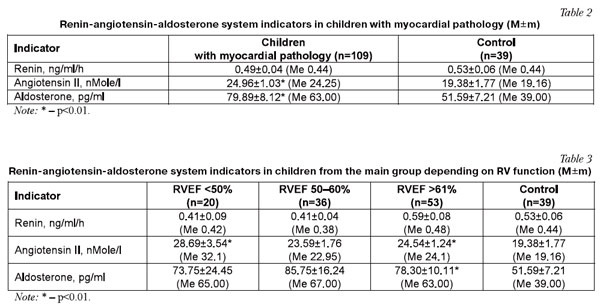
The studying of RAAS indicators cumulatively in the group revealed a verifiable increase of angiotensin II and aldosterone levels which fact attests to that system activation in children with MP (Table 2). However, RAAS activates differently in children with various levels of RV function; thus, children with RVEF>61% have demonstrated an insubstantial increase of plasma renin activity level with verifiable increase of angiotensin II and aldosterone levels (Table 3).
In children with moderate RVEF reduction (50.0-60.0%) further RAAS activation takes place, mostly due to aldosterone (Table 3).
At RV myocardium dysfunction progress (with EF lower than 50.0%) the tension in RAAS functioning increases, mostly at the cost of Рђ II (Table 3).
This way, with RV myocardium function preserved in children with MP, a compensatory activation of neurohumoral system takes place to sustain the haemodynamic balance. A progressive deconditioned RV remodelling with myocardium systolic function deterioration takes place on the background of more pronounced RAAS activation.
A correlation analysis of morphofunctional RV indicators and RAAS activity level showed no established correlation between them in the group in general. In the same time, a medium-strong direct correlation between the RVEF and aldosterone level (r=0.4; р<0.05) was established in children with RVEF >61%. In children with RVEF at 50.0–60.0%, a high level direct correlation between the RVEF and А II level (r=0.9; р<0.05) was found. The subgroup of children with RVEF less than 50% showed a high-level correlation between the RVMBV and aldosterone level (r=0.7, р<0.05) and between IEDD, IESD and А II (r=0.7; r=0.8; р<0.05). The revealed correlation links confirm a considerable effect the RAAS has onto development of deconditioned RV remodelling with its progressive dysfunction.
Conclusions
The systolic dysfunction of RV and its deconditioned remodelling in children with MP form and develop on the background of neurohumoral regulation systems (and in particular, RAAS) activation.
References
- Belenkov Yu.N., Ageev F.T., Mareev V.Yu. «Neyrogormoni i tcitokyni pry serdechnoy nedostatochnosty: novaya terapiya starogo zabolevaniya?». Serdechnaya nedostatochnost'. 2000; No.4: 1—6.
- Bogmat L.F., Rak L.I. Neyrogumoral'noe obespechenye funktcionyrovaniya serdechno-sosudistoy systemi u detey I podrostkov s patologiey miokarda. Ukr. kardiologich. jurn. 2006; Kardiologiya vchora, syogodni, zavtra: materiali mijnar. forumu, 17—19 trav. 2006 r.: 22—23.
- Voronkov L.G., Kovalenko V.N., Ryabenko D.V. Hronicheskaya serdechnaya nedostatochnost': mehanyzmi, standarti diagnostyki i lecheniya. K.: Morion, 1999: 120.
- Gorbachev V.A. Nedostatochnost' krovoobrashcheniya. — Minsk: Visheyshaya shkola, 1999: 590.
- Gurevich M.A. Hronycheskaya serdechnaya nedostatochnost':[ruk-vo dlya vrachey]. Рњ.: Praktuch. medetcina, 2008: 411.
- Dobrin B.Yu., Belaya I.E. Sovremennie predstavleniya o mehanizmah nachal'nih proyavleniy i progressyrovaniya serdechnoy nedostatochnosty. Ukr. kardiologich.jurn. 2005; No. 6: 143—150.
- Obrezan A.G., Vologdina I.V. Hronicheskaya serdechnaya nedostatochnost'. — SPb.: Bita Nova, 2002: 320.
- Cohn J.W., Ferrari R., Sharpe N. Cardiac remodeling — concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. On behalf of the International Forum of Cardiac Remodeling. J. Amer. Coll. Cardiol. 2000; 35: 569—582.
- Cowburn P.J., Cleland J.G. Endothelin antagonists for chronic heart failure: do they have a role? Erop. Heart. J. 2001; 19: 1772—1784.
- Nathan C. Qiao_wen Xie Regulation of Biosynthesis of Nitric Oxide. The Journal of Biological Chemistry. 1994; 269, No. 19: 13725—13728.
- Schrier R.W., Abraham W.T. Mechanisms of disease: Hormones and hemodynamics in heart failure. New Engl. J. Med. 1999; 341, No. 8: 577—585.
Эта статья с сайта:
Медицина. МедЭксперт
Постоянный адрес статьи:
http://old.medexpert.org.ua/modules/myarticles/article_storyid_949.html
Реклама:
Здоровая мания
Женское здоровье
Здоровье. Новости Украины
Здоровье. Главные новости
|


